Milk has an interesting behavior when you put soap in it:
Explanations of the chemistry behind this:
There are three chemicals involved:
Milk is a colloid — that is, the fat is clustered in globules. The homogenization of milk is done to reduce the size of these fat globules, with the intent of preventing or delaying the natural separation of fats from the rest of the emulsion.
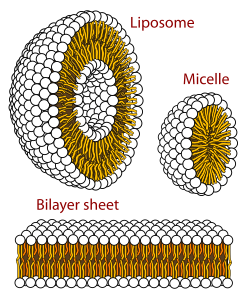
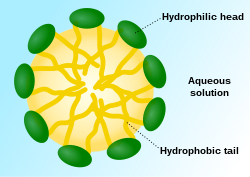
These fat globules are sometimes coated with a membrane of molecules that are polar on one side, and nonpolar on the other side. This configuration is called a micelle. (read more)
Thus, the fat globules are analogous to cell walls. Soap is similularly used to break down cell walls (eg. for DNA analysis).